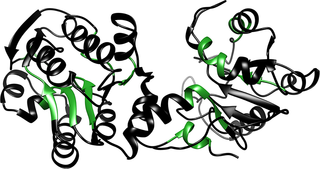
PGK is known to have ~7 such hydrophobic motifs that could potentially bind Hsp70, as shown in?Fig 9. These binding sites were predicted using the limbo website,?http://limbo.switchlab.org/limbo-analysis?[48], which was developed for DnaK (bacterial Hsp70), but is still likely useful for other Hsp70s, although it may not properly identify all Hsp70 binding sites in PGK. It has been observed previously that DnaK can bind near-native states of maltose binding protein and prevent unfolding with force [24]. Preemptive holdase activity in the cell similarly would enable Hsp70 to bind and hold substrate proteins prior to unfolding, stabilizing substrates against unfolding. We speculate that preemptive holdase activity may have evolved to preempt formation of misfolded or unfolded states of important substrates before they can have a chance to aggregate in the cell.
Hydrophobic patches are highlighted in green. These patches are potential binding sites for Hsp70 even when PGK is in its native state.