Brimrose AOTF-NIR光譜儀測定樹脂對硬化劑的百分比
I.? 簡介
聲光可調濾波器(AOTF)的原理基于光在各向異性介質中的聲折射。裝置由粘在雙折射晶體上的壓電導層構成。當導層被應用的射頻(RF)信號激發時,在晶體內產生聲波。傳導中的聲波產生折射率的周期性調制。這提供了一個移動的相柵,在特定條件下折射入射光束的部分。對于一個固定的聲頻,光頻的一個窄帶滿足相匹配條件,被累加折射。RF頻率改變,光的帶通中心相應改變以維持相匹配條件。
光譜的近紅外范圍從800nm到2500 nm延伸。在這個區域最突出的吸收譜帶歸因于中紅外區域的基頻振動的泛頻和合頻。是基態到第二激發態或第三激發態的能級躍遷。因為較高能級躍遷連續產生的概率較小,每個泛頻的強度連續減弱。由于躍遷的第二或第三激發態所需的能量近似于第一級躍遷所需能量的二倍或三倍,吸收譜帶產生在基頻波長的一半和三分之一處。除簡單的泛頻以外,也產生合頻。這些通常包括延伸加上一個或多個振動方式的伸縮。大量不同合頻是可能的,因而近紅外區域復雜,有許多譜帶彼此部分疊加。
現在,NIRS被用作定量工具,它依賴化學計量學來發展校正組成的參照分析和近紅外光譜的分析的關聯。近紅外數據的數學處理包括多元線性回歸法(MLR)、主成分分析法(PCA)、主成分回歸法(PCR)、偏最小二乘法(PLS)和識別分析。所有這些算法可以單獨或聯合使用來得到有價值組成的定性描述和定量預測。
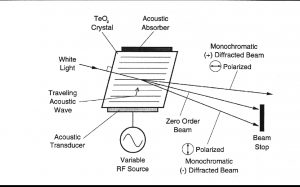
II.? 測量方法
?
- Sample Preparation
Brimrose was supplied with approximately 300ml of a polyester resin solution labeled COAT1 and approximately 100 ml of a polyisocyanate solution labeled CO-REACTANT 1.? Because both solutions were too viscous to be drawn through a measuring pipette, an alternate method for measuring out proportions was used.? Using a measuring pipette, 10mL of ethyl acetate was measured and dispensed into a small 15mL vial and the level obtained was marked.? This procedure was repeated for each vial.? Each vial was then filled to this mark with the polyester resin.? A similar procedure was used to measure the more viscous co-reactant.? The ends of the plastic pipette tips were enlarged by clipping off approximately 5mm of the tip, so that the co-reactant could be drawn into them.? An amount of ethyl acetate was drawn into the pipette tip and the level marked.? This was done for each sample prepared as a secondary assurance that the proper amount of co-reactant was being drawn into the pipette tip (see Tables 1 and 2 for prepared samples).? Twenty five percent of the co-reactant is ethyl acetate.? In order to obtain a true percent volume of the co-reactant in each sample the 25% ethyl acetate was first factored out.? The product was then divided by the total volume and multiplied by a constant of 10.?? A chemical reaction begins to take place upon mixing of the two solutions and spectra were immediately collected.
| Sample | COAT1 | Co-reactant | % Co-reactant |
| S-01 | 10ml | 0.600ml | 0.42 |
| S-02 | 10ml | 0.550ml | 0.39 |
| S-03 | 10ml | 0.530ml | 0.38 |
| S-04 | 10ml | 0.500ml | 0.36 |
| S-05 | 10ml | 0.480ml | 0.34 |
| S-06 | 10ml | 0.450ml | 0.32 |
| S-07 | 10ml | 0.430ml | 0.31 |
| S-08 | 10ml | 0.400ml | 0.29 |
| S-09 | 10ml | 0.380ml | 0.27 |
| S-10 | 10ml | 0.350ml | 0.25 |
| S-11 | 10ml | 0.330ml | 0.24 |
| S-12 | 10ml | 0.300ml | 0.22 |
| S-13 | 10ml | 0.280ml | 0.20 |
| S-13A | 10ml | 0.280ml | 0.20 |
| S-14 | 10ml | 0.250ml | 0.18 |
| S-14A | 10ml | 0.250ml | 0.18 |
| S-15 | 10ml | 0.550ml | 0.39 |
| S-16 | 10ml | 0.600ml | 0.42 |
| S-16A | 10ml | 0.600ml | 0.42 |
Table 1.?? Calibration Set
| Sample | COAT1 | Co-reactant | % Co-reactant |
| VAL-17 | 10ml | 0.570ml | 0.40 |
| VAL-18 | 10ml | 0.520ml | 0.37 |
| VAL-18 | 10ml | 0.520ml | 0.37 |
| VAL-19 | 10ml | 0.460ml | 0.33 |
| VAL-19 | 10ml | 0.460ml | 0.33 |
| VAL-20 | 10ml | 0.4150ml | 0.30 |
| VAL-20 | 10ml | 0.415ml | 0.30 |
Table 2.? Validation Set
- Data Collection
A Brimrose AOTF-NIR Luminar spectrometer was used for data collection.? Spectra were collected in transmission mode using fiber optic cable with 600mm diameter low OH Silica core.? The spectral range was 900nm to 1700nm with a 2nm resolution.? The effective path-length was 10mm.? Spectra were also collected on the raw solutions.
III. Results
- Spectra
?????????? The absorbance spectra of the raw solutions are shown below in Figure 2. ?These spectra show clearly the areas of each solution that absorb differently.? Figure 2 shows that the co-reactant absorbs quite strongly between 1450nm and 1600nm, while the polyester resin has no absorbing properties in the same region.? This will prove to be an important region when modeling the data and will be discussed later in this report.? There is a slight difference between the absorbing characteristics of the resin and the co-reactant at 1384nm.? It is clear that the resin has a stronger absorbing peak at 1177nm.?? These two areas of the spectral range will also show to be important when modeling the data.
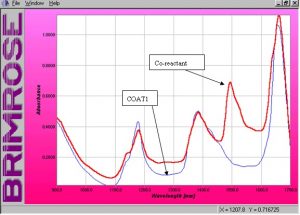
Figure 2.? Absorbance spectra of raw solutions
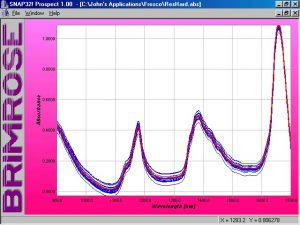
Figure 3.? Absorbance spectra of mixed COAT1 and co-reactant solutions
Initially 16 samples were prepared.? An aliquot of samples S-13, S-14, and S-16 were scanned and added into the calibration for a combined total of 19.? Spectra of the 19 samples are shown in Figure 3.? Later 4 additional samples with proportions not represented in the calibration set were prepared and scanned.? An aliquot of VAL-18, VAL-19 and VAL-20, for a total of 7 samples in the validation set, were also scanned and included in the total matrices (see Table 2).
- Modeling and Regressions
There were a total of 26 spectra, which were imported as absorbance data into the chemometrics software package The Unscrambler?.? As stated earlier in this report, the complete wavelength range was from 900nm to 1700nm.? For modeling purposes, different wavelength regions were examined in an attempt to obtain the best results.? After analyzing the spectra and performing regressions on several different regions, the region between 1172nm and 1620nm was found to give the best results.? Sample S-03 was considered a highly influential outlier due to both high residual Y variance and leverage and was not included in the regression.? The regression is shown below in Figure 4.? With sample S-03 removed, the regression shows very good correlation between X (spectra) and Y (response or measured) data.? This holds true for both the calibration and validation.? Correlation values were 0.97 and 0.95 respectively.? The SEP (standard error of prediction) and SEC (standard error of calibration) was 0.028 and 0.022 respectively.
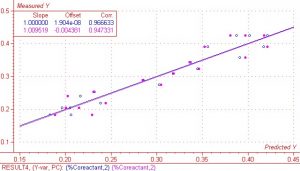
Figure 4.? Regression on % co-reactant with one outlier removed.
As previously mentioned, the region between 1450nm and 1600nm is an area in which the COAT1 solution does not absorb and the co-reactant absorbs quite strongly.? This same wavelength region results in very high regression coefficients and X loading, which indicates there is information in the spectral data that correlates well to the measured Y response values.? Regression coefficients and X loading are shown below in Figures 5 and 6 respectively.? Although not quite as pronounced, the regression coefficients and X loading are slightly higher in the other two spectral regions of interest, i.e. 1384nm and 1177nm.
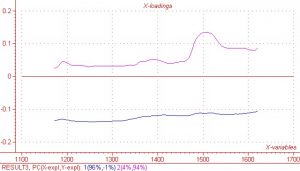
Figure 5.? X – Loading for PC’s 1 and 2
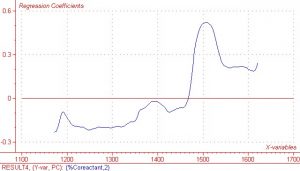
Figure 6.? Regression coefficients for second Principle Component
- Prediction
A model built from 18 calibration samples, which excluding sample S-03 was applied to the 7 samples mixed and retained for validating the model. These samples are identified as such in Table 2. The SEP for the Validation set was 0.026 and agrees quite closely with 0.027 given for the model.? Prediction results can be seen in Table 3 below.
| ????? % Co-Reactant by Volume | |||
| Sample | Predicted | Measured | Difference |
| VAL-17 | 0.414 | 0.40 | 0.010 |
| VAL-18 | 0.361 | 0.37 | -0.010 |
| VAL-18 | 0.358 | 0.37 | -0.013 |
| VAL-19 | 0.342 | 0.33 | 0.012 |
| VAL-19 | 0.392 | 0.33 | 0.062 |
| VAL-20 | 0.336 | 0.30 | 0.037 |
| VAL-20 | 0.308 | 0.30 | 0.009 |
| SEP= | 0.026 | ||
Table 3.? Prediction result on % co-reactant
- Conclusion
The results of this feasibility study indicate that the determination of? % co-reactant in a mixture with COAT1 is very feasible using the Brimrose AOTF-NIR Luminar spectrometer.? However, better results could be obtained by using a larger calibration set of approximately 40 samples in a laboratory with tools that allow for more accurate measurements.? There were many unavoidable errors because of the limited resources available for doing this experiment.? This is especially true for the more viscous co-reactant.? It was necessary to first measure a volume of solvent and then pour this amount in a vial.? The volume amount was then marked on the vial.? The COAT1 was then poured into the vial and brought to the mark.? This two step process increased the amount of error associated with measuring precise amounts.? The same procedure was used for the more viscous co-reactant but the error factor was even greater.? A certain amount was lost because the pipette tip could not be completely rinsed of the co-reactant.? Another error factor was that a given amount of the co-reactant clung to the outside of the pipette and was unavoidably dissolved into the mixture.? A third way of introducing error was in the size of the samples.? Because none of the samples was greater than 10.6mL, any error in measurement will by compounded by the use of such small samples.? Making corrections to the error factors in this experiment should result in obtaining a more precise calibration set which will create a more robust model that is able to predict with greater accuracy.



