Brimrose AOTF-NIR光譜法鑒別乳液和懸浮聚氯乙烯PVC粉末
I. 操作總結
Brimrose AOTF-NIR Luminar 2030自由空間光譜儀用于掃描2種不同種類聚氯乙烯(PVC)粉末的光譜數據,產品的商業名稱是Lztavil,兩種類型的PVC粉末是懸浮物和乳液。結果表明,光譜差異清晰明了,表明Brimrose自由空間光譜儀可以區分這兩種PVC粉末。PCA分析驗證了Brimrose自由空間光譜儀能夠使用光譜數據和PCA校準模型準確確定正在掃描的粉末類型。
II. 簡介
聲光可調濾波器(AOTF)的原理基于光在各向異性介質中的聲折射。裝置由粘在雙折射晶體上的壓電導層構成。當導層被應用的射頻(RF)信號激發時,在晶體內產生聲波。傳導中的聲波產生折射率的周期性調制。這提供了一個移動的相柵,在特定條件下折射入射光束的部分。對于一個固定的聲頻,光頻的一個窄帶滿足相匹配條件,被累加折射。RF頻率改變,光的帶通中心相應改變以維持相匹配條件。
光譜的近紅外范圍從800nm到2500 nm延伸。在這個區域最突出的吸收譜帶歸因于中紅外區域的基頻振動的泛頻和合頻。是基態到第二激發態或第三激發態的能級躍遷。因為較高能級躍遷連續產生的概率較小,每個泛頻的強度連續減弱。由于躍遷的第二或第三激發態所需的能量近似于第一級躍遷所需能量的二倍或三倍,吸收譜帶產生在基頻波長的一半和三分之一處。除簡單的泛頻以外,也產生合頻。這些通常包括延伸加上一個或多個振動方式的伸縮。大量不同合頻是可能的,因而近紅外區域復雜,有許多譜帶彼此部分疊加。
現在,NIRS被用作定量工具,它依賴化學計量學來發展校正組成的參照分析和近紅外光譜的分析的關聯。近紅外數據的數學處理包括多元線性回歸法(MLR)、主成分分析法(PCA)、主成分回歸法(PCR)、偏最小二乘法(PLS)和識別分析。所有這些算法可以單獨或聯合使用來得到有價值組成的定性描述和定量預測。
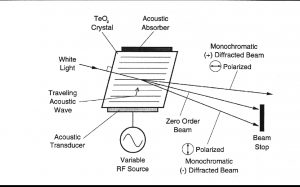
Figure 1.? Schematic of the AOTF
III.? 方法論
A Brimrose AOTF-NIR Luminar 2030 Free Space spectrometer was used to scan spectral data on 2 different types of PVC powder.? Spectra were first collected for the emulsion powder and then for the suspension powder.? The powders were placed in a dish and spectra were collected in two different ways for both powders.? The first method of spectra collection was to not move the dish at all during data collection, resulting in the collection of data when the PVC powder is static.? The second method was to move the dish around as the powder was scanned, resulting in the dynamic data collection of PVC powder.? The powder was measured to keep it at the optimum distance of 40mm from the optical module of the Free Space spectrometer.? The spectra were collected in ratio mode to account for any fluctuations in the lamp.? Wavelength range was from 1200nm to 2300nm with 2nm resolution.? 100 scans were collected per reading and averaged into one spectrum.? Data acquisition time was about 5 seconds per spectrum.? 25 individual spectra were collected for both the emulsion powder and the suspension powder during static and dynamic data collection.? Raw spectral data was collected in transmission mode and post-processed into absorbance and first derivative.? PCA analysis was performed to test the feasibility of classifying the two types of powder based on spectral data.
IV. Results
- Spectra
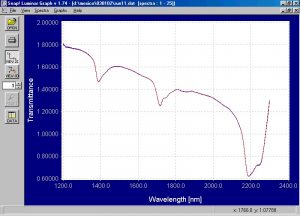
Figure 2.? Raw transmission static spectra of emulsion PVC powder.
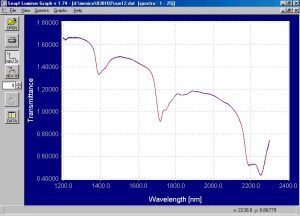
Figure 3.? Raw transmission static spectra of suspension PVC powder.
The raw transmission static spectra of both the emulsion and suspension PVC powder shows good signal to noise ratio.? The transmission static spectra of the emulsion and suspension PVC powder were processed into absorbance and merged into one file for comparison purposes.
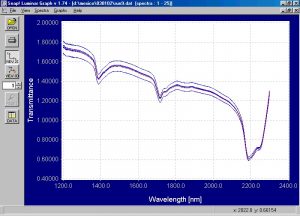
Figure 4. ??Raw transmission dynamic spectra of emulsion PVC powder.
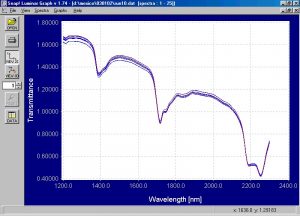
Figure 5.? Raw transmission dynamic spectra of suspension PVC powder.
It is clear that a small baseline shift is occuring due to the movement of the powder during dynamic spectra collection.? The chemometric modeling is not affected by a baseline shift and any small variations in distance from the powder sample to the optical head of the Free Space spectrometer will not affect the ability to distinguish between emulsion and suspension PVC powder.? The first derivative spectra eliminate any baseline shift effects and the first derivative spectra for both types of powder will show this.? The raw dynamic transmission spectra was processed into absorbance and merged into one file for comparison purposes.
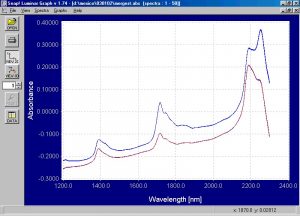
Figure 6.? Static absorbance spectra of emulsion and suspension PVC powder.
There are clear and discernible spectral differences between the emulsion and suspension PVC powder.? Absorbance peaks are shown around 1400nm, 1700nm, and 2200 nm.? There is a difference in the absorbance peaks around 1700nm, which is a common absorbing area of the spectrum for polymers due to the presence of C-H bonds.? There is also a very clear difference in the spectra from 2200nm to 2300nm.? The spectra shown here certainly indicate that the Brimrose Free Space spectrometer can distinguish between the two types of PVC powder and the PCA modeling will confirm this.? The results for the dynamic spectra are similar and are not shown here.? The spectra in the wavelength areas of interest were enhanced and shown in the following graphs.
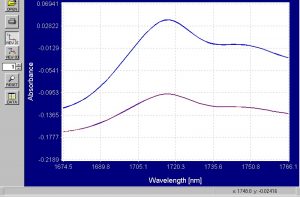
Figure 7.? Enhanced absorbance spectra of emulsion and suspension PVC powder from 1674nm to 1766nm.
There is a very clear difference in the amplitude of the absorbance peaks that occur around 1715nm.? The absorbance peak for the suspension PVC powder is much larger.? This difference alone is enough for the Brimrose Free Space spectrometer to distinguish between the 2 types of powders and the PCA modeling will prove this.? The differences in the peaks at 2200nm are even larger and this is shown in the graph below.
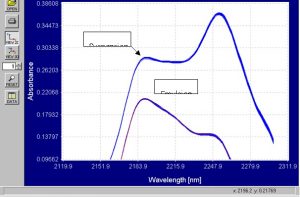
Figure 8.? Enhanced absorbance spectra of emulsion and suspension PVC powder from 2120nm to 2300nm.
There are very clear differences in the spectra of both the powders from 2200nm to 2300nm.?? The emulsion spectra levels out and the absorbance goes down after 2200nm.? The suspension spectra levels out at 2200nm and there is a peak that is not present in the emulsion spectra around 2250nm.? These spectra contain more than enough information for the Brimrose Free Space spectrometer to separate emulsion and suspension powder based on spectral data and the chemometric modeling will prove this.? Spectra were processed from absorbance into first derivative and these spectra are shown in the graphs below.
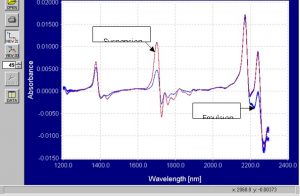
Figure 9.? First derivative static spectra of emulsion and suspension PVC powder.? ??
The first derivative dynamic spectra of the emulsion and suspension powders are shown in the graph below for comparison purposes.
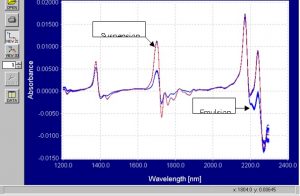
Figure 10.? First derivative dynamic spectra of emulsion and suspension PVC powder.
The first derivative static and dynamic spectra are virtually identical and the baseline shift effect in the absorbance spectra is eliminated.? There are very clear spectral differences around 1450nm, 1700nm, and 2250nm.? The first derivative spectra often decrease the signal-to-noise ratio but the effect is very small here.? Maintaining high signal-to-noise ratio in the first derivative spectra is an indication of good, robust raw data.? The differences around 2250nm are similar to those in the absorbance spectra.? The differences around 1450nm and 1700nm are different from those in the absorbance spectra and those differences are enhanced in the graphs below.
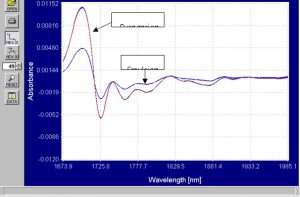
Figure 11.? Enhanced first derivative spectra from 1673nm to 1985nm.
The differences in the first derivative spectra in these graphs are very pronounced and provide more than enough information for separation of emulsion and suspension PVC powders.? It is often the case that using first derivative spectra for modeling is preferable when the differences between two compounds are very small.? In this case, the necessary separation is of two polymer powders of the same material but in different forms.? It is necessary to maintain good signal-to-noise ratio when using first derivative spectra and the spectra collected in this study indicate that the first derivative spectra could be used for modeling.? PCA models were created using both the absorbance and first derivative spectra.
- Regressions and Modeling
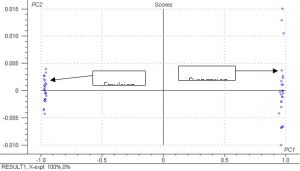
Figure 12.? Scores plot from PCA analysis for emulsion and suspension absorbance spectra.
compounds based on spectral data.? The scores plot shows how well classification can occur based on spectral data and it is clear that the differences in the spectra are enough to?? distinguish between emulsion and suspension PVC powder.? Only one principle component was used for the separation, which indicates that the model is able to separate the powders very easily based on the spectral data.? This analysis was performed using the static absorbance spectra.? The results were similar for the dynamic spectra and are not shown here.? Any change in signal intensity will not affect classification results.? First derivative spectra eliminates the baseline shift effect altogether and the scores plot for the PCA analysis based on the first derivative spectra is shown below.
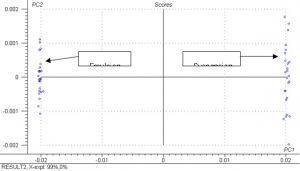
Figure 13.? Scores plot from PCA analysis for emulsion and suspension first derivative spectra.
The results for the PCA analysis are excellent and clearly show that emulsion and suspension PVC powder can be separated based on spectral data.? The PCA analysis for both the absorbance and first derivative spectra indicate that either can be used for separating the emulsion and suspension PVC powders.
V. Discussion and Conclusions
The results of this study clearly indicate that it is feasible to separate emulsion and suspension PVC powders based on spectral data obtained using the Brimrose AOTF-NIR Luminar 2030 Free Space spectrometer.? Despite the fact that the 2 compounds being separated are the same PVC polymer powder in different forms, the spectral differences are clear and discernible.? The Brimrose AOTF-NIR spectrometer is the ideal tool for an on-line industrial process because of its high scanning speed, low signal-to-noise ratio, and lack of moving parts.? The AOTF technology would allow for real-time on-line detection of emulsion and suspension PVC powder at a rate of about 5 seconds per reading.? The Free Space optical head can be mounted above the process line and no contact will be made with the powder.? Past experience has shown that a calibration model created from laboratory data can easily be transferred to a real-time, on-line situation.? Classification is relatively simple in this case and there is no question that a calibration model can be created in the laboratory and then used to distinguish between powder types in the industrial process line.? The spectral differences are more pronounced in the first derivative spectra but it is important to maintain good signal-to-noise ratio.? In any case, the spectral differences are large enough in both the absorbance and first derivative spectra that both types of spectra can be used for modeling.? Overall, the results of this study were excellent and clearly proved that emulsion and suspension PVC powders can be separated using spectral data collected using the Brimrose AOTF-NIR Free Space spectrometer.



