UV-vis spectrophotometry on intact, active cells is notoriously difficult, due to the scatter of measurement light by cell suspensions. For decades, the first step in examining the chromophores of the ETC was to disrupt the cells and separate the contents. Today, research using a new integrating cavity spectrometer has demonstrated that isolated cell components do not act the same as when those components are in their native environment, for example, when they are not in the environment in which they were designed to function, they may behave differently.
The data presented in this article includes spectra of the ETC in baker’s yeast as the cells dramatically alter in response to the removal of oxygen from their environment as the yeast metabolize endogenous substrates. Similar studies have been conducted using mammalian cells, bacteria, algae, and other intact, non-disrupted systems and can be seen elsewhere.
The CLARiTY spectrometer features an 8 mL cuvette that exhibits an effective pathlength approaching 30 cm, thereby providing exceptionally high sensitivity. The instrument used here is the OLIS RSM 1000 based CLARiTY, which scans a full 220 nm width spectrum of the ETC 100 times per second.
Experimental
Yeast suspended in PBS (3 mg/mL, or ~6 × 107?yeast cells/mL) were loaded into the CLARiTY’s integrating cavity and a baseline was collected over several seconds. The instrument acquired three scans per second for 2 min. Figure 1a shows the cells the were used, photographed in a conventional 1 cm2?cuvette; Figure 1b displays some of the spectra as the yeast became increasingly hypoxic by consuming the available oxygen.
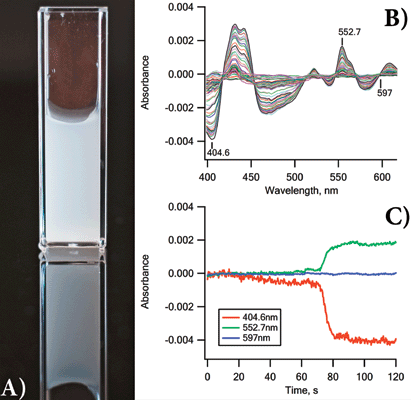
Figure 1: (a) Photograph of the yeast suspension used in this experiment. Meaningful UV-vis data from such a suspension in traditional 1 cm2?cuvette-based spectrometers are impossible to obtain; (b) 20 of the absorbance scans acquired as the oxygen level dropped; (c) kinetics of the spectral changes of the yeast ETC at three key wavelengths.
Features of all the expected chromophores (cytochrome c, b, a3, etc.) appeared and changed as the oxygen concentration fell to zero. Kinetic changes at three wavelengths are shown as Figure 1c.
Following the ETC as it responds to different environmental conditions using intact and active cells instead of purified chromophores represents an important advance in the research of in situ systems using UV-vis absorbance spectroscopy.
Results
Figure 1b shows absorbance spectra collected to record the changes occurring in the ETC as the yeast metabolized the available endogenous substrates and consumed the oxygen. Slow changes were recorded over about 90 s and then rapid changes in the spectra occurred over 10 s, as the yeast cells became hypoxic.
Conclusion
The OLIS CLARiTY spectrophotometers provide biologists, biochemists, microbiologists, and those who study mitochondria a means to obtain high quality, high sensitivity, and accurate UV-vis spectra, so as to follow metabolic and bioenergetic changes occurring in living, physiologically sound experimental samples.
References
(1) “In situ Spectroscopy on Intact Leptospirillum Ferrooxidans Reveals that Reduced Cytochrome 579 is an Obligatory Intermediate in the Aerobic Iron Respiratory Chain.”?Front Microbiol, 2012 Apr. 12;3:136. doi: 10.3389/fmicb.2012.00136, eCollection 2012.
(2) “Interaction of the carbon monoxide-releasing molecule Ru(CO)3Cl(glycinate) (CORM-3) with Salmonella entericaserovar Typhimurium: in situ measurements of CO binding by integrating cavity dual beam spectrophotometry,”?Microbiology?mic.0.081042-0; published ahead of print August 1, 2014.
(3) “Rising CO2?Interacts with Growth Light and Growth Rate to Alter Photosystem II Photoinactivation of the Coastal Diatom Thalassiosira Pseudonana,”?PLoS ONE?8(1): e55562. doi:10.1371/journal.pone.0055562.
Olis, Inc.
130 Conway Drive, Suites A, B & C, Bogart, GA 30622
tel. (800) 852-3504, (706) 353-6547
Website:?www.olisweb.com