Interaction of the GTPase Elongation Factor Like-1 with the Shwachman-Diamond Syndrome Protein and Its Missense Mutations
GTP酶伸長因子Like-1與Shwachman-Diamond綜合征蛋白的相互作用及其錯義突變
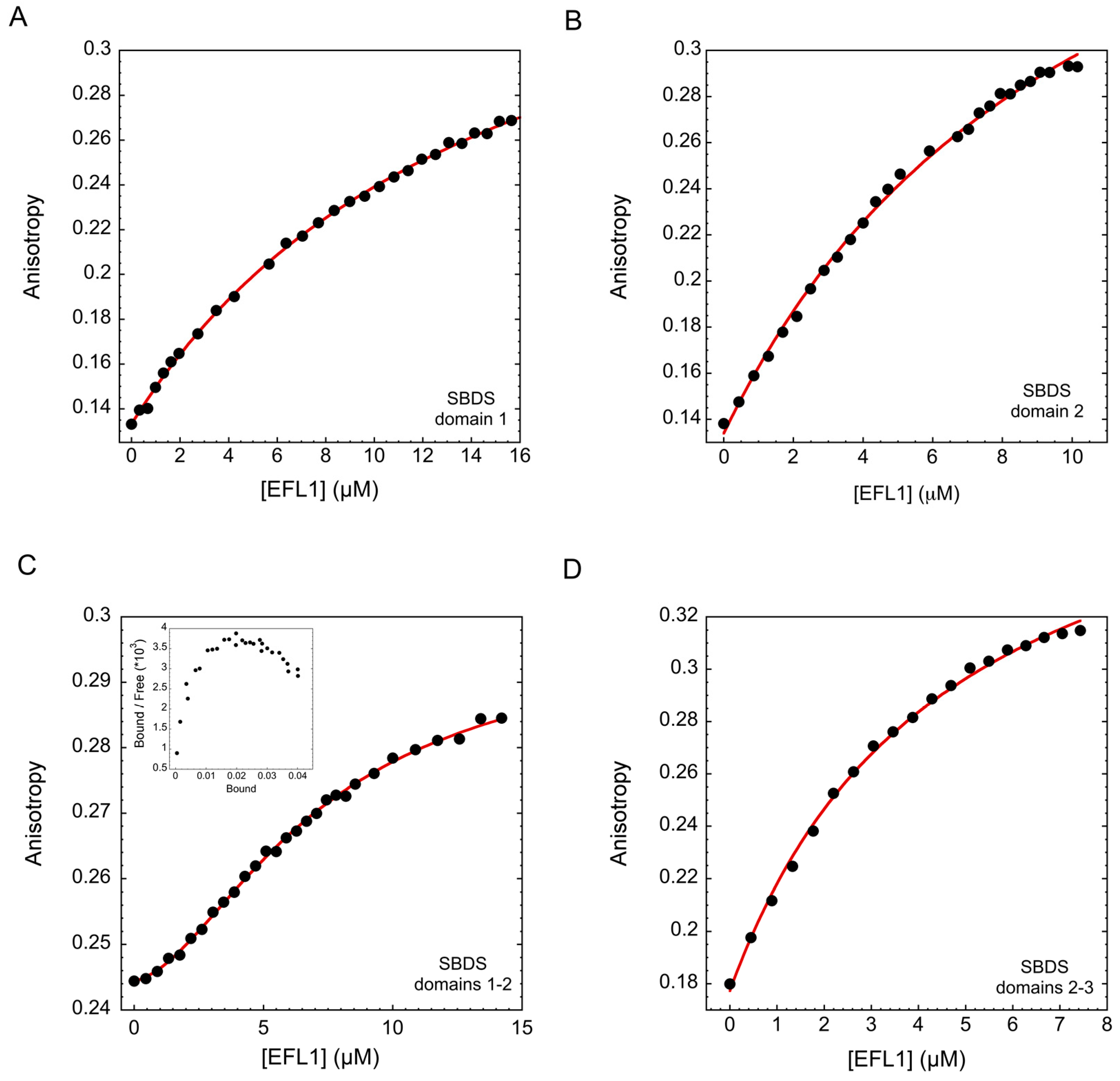
The Shwachman-Diamond Syndrome (SDS) is a disorder arising from mutations in the genes encoding for the Shwachman-Bodian-Diamond Syndrome (SBDS) protein and the GTPase known as Elongation Factor Like-1 (EFL1). Together, these proteins remove the anti-association factor eIF6 from the surface of the pre-60S ribosomal subunit to promote the formation of mature ribosomes. SBDS missense mutations can either destabilize the protein fold or affect surface epitopes. The molecular alterations resulting from the latter remain largely unknown, although some evidence suggest that binding to EFL1 may be affected. We further explored the effect of these SBDS mutations on the interaction with EFL1, and showed that all tested mutations disrupted the binding to EFL1. Binding was either severely weakened or almost abolished, depending on the assessed mutation. In higher eukaryotes, SBDS is essential for development, and lack of the protein results in early lethality. The existence of patients whose only source of SBDS consists of that with surface missense mutations highlights the importance of the interaction with EFL1 for their function. Additionally, we studied the interaction mechanism of the proteins in solution and demonstrated that binding consists of two independent and cooperative events, with domains 2–3 of SBDS directing the initial interaction with EFL1, followed by docking of domain 1. In solution, both proteins exhibited large flexibility and consisted of an ensemble of conformations, as demonstrated by Small Angle X-ray Scattering (SAXS) experiments.?View Full-Text
https://www.mdpi.com/1422-0067/19/12/4012